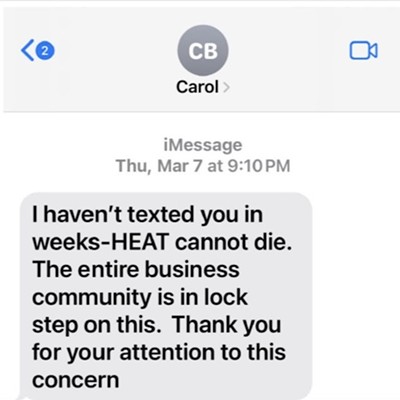
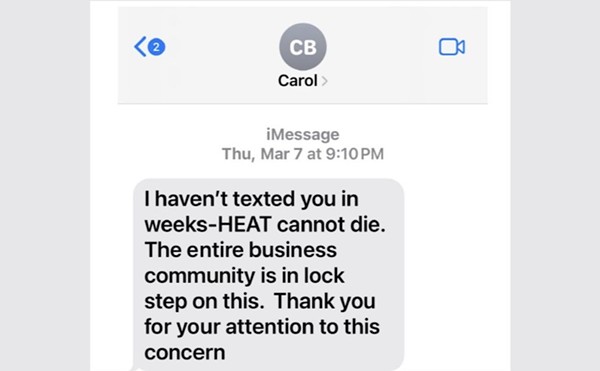
Anyone even vaguely familiar with cannabis law knows it's fraught with complications and contradictions. Now, in light of recent legal developments, the legal status of cannabidiol, commonly known as CBD, the non-psychoactive medicinal compound derived from cannabis, has never been hazier.
The federal farm bill being considered by Congress could legalize hemp – the Senate version passed 86-11 on June 28 does, but the House version passed June 21 doesn't mention hemp at all. The two versions will have to be reconciled before the bill can go to the Oval Office for President Donald Trump's signature.
But, when combined with the U.S. Food and Drug Administration's approval of the first-ever pharmaceutical drug containing CBD, the farm bill may actually pave the way for Big Pharma to take over the CBD market – and the rest of the medical marijuana market – nationwide.
On June 25, the FDA approved London-based GW Pharma's Epidiolex, a drug containing CBD as its active ingredient, to treat two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients 2 years old and older. On the surface, that may seem like a victory for medical marijuana advocacy but, in reality, it only further muddies the legal status of CBD.
In accordance with the Federal Food, Drug and Cosmetic Act, the FDA considers any product for human consumption containing the active ingredient (e.g., CBD) of an FDA-approved pharmaceutical drug (e.g., Epidiolex) to be "adulterated and misbranded," according to a Q&A posted on the FDA website.
"There is an exception," the FDA Q&A states, "if the substance was 'marketed as' a dietary supplement or as a conventional food before the drug was approved. ... However, based on available evidence, FDA has concluded that this is not the case for THC or CBD. ... Interested parties may present the agency with any evidence that they think has bearing on this issue."
Jonathan Miller, lead counsel for the U.S. Hemp Roundtable, a coalition of dozens of hemp companies and one of many hemp industry lobbying groups, says he's been gathering such evidence and hopes to change the agency's mind.
But for the time being, the FDA's press release announcing the approval of Epidiolex made it clear they aren't backing down from that position.
"[W]e are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims," it reads in part. "Marketing unapproved products, with uncertain dosages and formulations, can keep patients from accessing appropriate, recognized therapies to treat serious and even fatal diseases."
In fact, Stanley Brothers, the Colorado cannabis company best known for their high-CBD cannabis strain, Charlotte's Web, credited with helping children control seizures when conventional pharmaceuticals failed, received a warning letter from the FDA last year regarding the medical claims made about their CBD products. Stanley Brothers is one of the member companies represented by the Roundtable.
Now that Epidiolex has been approved, the FDA will prepare a "medical and scientific analysis" of the new drug for the U.S. Drug Enforcement Agency, which may then choose to move CBD from Schedule I in the Controlled Substances List to Schedule II-V, meaning only pharmaceutical companies, doctors and pharmacists will be allowed to legally produce, prescribe and distribute CBD, respectively, in compliance with federal law. Every step of the process would have to comply with FDA standards, and everyone in the supply chain (growers, extractors, retailers, etc.) would have to be registered with the DEA.
The DEA could also choose to create a new drug code exclusively for Epidiolex under Schedule II-V, DEA spokesperson Barbara Carreno explains, leaving all other forms of CBD just as federally illegal as they are now. That's how the DEA chose to deal with Marinol, an FDA-approved drug containing synthetic THC, the main psychoactive compound in cannabis, and it could do the same with any other cannabis-based drugs the FDA approves in the future.
Carreno says, "There is precedent for regulating various compounds derived from the same plant differently," noting the various opioids derived from poppies range from heroin in Schedule I to oxycodone and morphine in Schedule II to codeine cough syrups in Schedule V.
The only thing that's certain is that Epidiolex will not go in Schedule I, because the FDA has confirmed that it has medical uses. Controlled substances in Schedule II through V are classified according to their risk of abuse and overdose, with Schedule II having the highest risk and V having the least.
But Colorado hemp farmer and activist Veronica Carpio believes the FDA approval of Epidiolex could give the federal government the strongest legal grounds yet to justify a crackdown on CBD (and cannabis in general) while simultaneously laying the groundwork for Big Pharma to take over the medical marijuana industry nationwide.
According to Justia.com, which makes legal records available to the public, U.K. pharmaceutical company GW also holds roughly 100 patents related to the medical efficacy of cannabis, covering cannabinoid extraction processes, cannabinoid placebos, dispensing mechanisms for cannabinoid drugs, and a range of cannabinoid-based drug formulas used to treat almost everything under the sun, including schizophrenia, mood disorders, epilepsy, Alzheimer's disease, psychosis, bone disorders, inflammatory bowel diseases (e.g., Crohn's disease), tumors, bulimia, obesity and several types of cancer. Each patent potentially provides another legal avenue for GW to shut down its competitors already operating legally.
Industry website the Cannabist reports, "Forecasts have put Epidiolex's peak annual sales between $1 billion and $3 billion." Epidiolex is expected to cost between $2,500 and $5,000 per month, according to analysts consulted by the New York Times, yet Colorado medicinal hemp farmer Jan VanDenBerg says her products could deliver the same dosage of CBD for roughly a tenth of those prices. Unlike other CBD and MMJ products, however, Epidiolex may be covered by private health insurance – but the ever-rising costs of insurance may only create another barrier to access.
Fortunately for Colorado's CBD businesses, the Colorado Hemp Foods Bill was signed into law earlier this year, and it takes the exact opposite position of the FDA, asserting explicitly that CBD is a dietary ingredient and products containing it should be regulated as food products.
Carpio, who helped draft and advocate for the bill, says it could be a turnkey solution for all the other states (like Florida, if we ever get there) to protect their CBD markets from the feds.
Defense attorney and first chair of the Colorado Bar Association's Cannabis Law Committee Lenny Frieling says the law may protect Colorado from an FDA or DEA crackdown on CBD on the basis of states' rights, but it could take a lawsuit, or several, to finally sort it out.
The difference between regulating CBD as a drug or a food is a massive one for farmers. Folium Biosciences spokesperson Juanita Ramos helped advocate for the Hemp Foods Bill and agrees with Carpio that CBD should be regulated as a food product, but Folium's leadership is prepared for any eventuality.
"All we can do is keep our heads down and keep doing what we do," she says. "We're concentrating on those kids and people who need [CBD]."
All the farmers we interviewed agree that, rather than being put in a new drug schedule, CBD should be removed from the list of Controlled Substances entirely because it carries little to no risk of abuse or overdose.
"Descheduling will really open it up to anyone who wants to participate, and they wouldn't have burdensome requirements that they have to follow because it will be treated as a regular agricultural crop," Luke Johnson, CEO of Cloud CO Farms in Alamosa, Colorado, says. "That would finally allow farmers and producers to operate in a more fair and competitive landscape to all the international people that we compete with currently."
Johnson hopes the upcoming farm bill will accomplish just that. The Senate version of the bill contains an amendment to federally legalize hemp proposed by Senate Majority Leader Mitch McConnell, R-Kentucky.
"I have heard from many Kentucky farmers who agree it's time to remove the federal hurdles and give our state the opportunity to seize its full potential and once again become the national leader for hemp production. That is why I strongly advocated for this measure to be included in the Farm Bill," McConnell said in a statement after the farm bill passed the Senate.
Technically, McConnell's amendment removes "hemp" from the definition of "marihuana" in the Controlled Substances Act, and defines hemp as "the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis."
The Hemp Roundtable's Miller and Colleen Lanier, a spokesperson for the Hemp Industries Association, another trade group, believe that language should be sufficient to protect all hemp-derived CBD products from federal interference.
Carpio disagrees, however, pointing out a paragraph of the amendment added in committee: "(b) EFFECT ON OTHER LAW. Nothing in this subtitle shall affect or modify (1) the Federal Food Drug and Cosmetic Act (21 U.S.C. 301 et seq.); or (2) the authority of the Commissioner of Food and Drugs and the Secretary of Health and Human Services under that Act."
Combined with the approval of Epidiolex, Carpio says that paragraph turns the bill from a victory gift for the CBD industry into a Trojan Horse. Defense attorney and first chair of the Colorado Bar Association's Cannabis Law Committee Frieling agrees with Carpio's interpretation of that paragraph, but says it's impossible to know exactly how the FDA will go about enforcing the FD&C Act in regard to CBD.
Miller, an attorney, believes the FDA's enforcement efforts regarding CBD will only be focused on companies making unproven medical claims about their products, which he fully supports, rather than a full-blown crackdown.
"I just don't see the FDA getting into that battleground," Miller says. He believes there should be two paths available for consumers to access CBD – as a pharmaceutical drug or as an over-the-counter food product or supplement – and if the FDA tries to block the latter path, he and the Roundtable will be prepared to sue.
But Carpio says the FDA wouldn't have to crack down on, nor would GW have to sue, every company making CBD products – going after a few big companies, like Stanley Brothers, could be enough to scare off smaller ones that can't afford to get tied up in litigation.
"This is going to change people's lives for the better everywhere," Dani Billings, a hemp farmer and co-founder of the Colorado Hemp Project, says.
Billings believes, if federally legalized, hemp could be the crop that brings success and stability back to family farms across the country, especially because of its versatility. Hemp can be used to produce everything from biodegradable plastics and building materials to textiles and rope to food, medicine and biofuels.
"This plant is here to do good – to be a positive conscious force," Billings says. "Everybody has health issues. Everybody needs houses. Everybody needs clothes, and everybody needs food. And this plant can take care of all of that. Once this plant has an opportunity to touch people's lives, Lord does it make a change."
Billings was one of the first Colorado farmers to begin growing hemp after the 2014 farm bill authorized states to create industrial hemp programs for research. Today, over 40 states, including Florida, have passed legislation creating such programs – 19 are now actually producing hemp, according to Lanier at the Hemp Industries Association.
Hemp became legal to grow in Florida in July 2017. According to Robert Gilbert, professor and chairman of the agronomy department at the UF Institute of Food and Agricultural Sciences, the plant has not been legally grown in the state since before World War II. The Florida Department of Agriculture & Consumer Services passed its permitting process on April 12 of this year, and UF-IFAS researchers are now set to launch a pilot program to help the state develop the industry.
Despite hemp's relative success, because of its Schedule I status, those in the industry still face unique challenges and restrictions. Billings and Johnson say they have been denied banking services and credit card processing, had multiple bank accounts shut down on them, and they pay extra for the accounts they do have because they are considered high-risk. Plus, nothing they grow, produce or sell can cross state lines or they risk having it seized by the DEA. All this for a plant that doesn't get you high.
Perhaps the most important benefit hemp farmers will receive if the Senate's farm bill becomes law is access to federal crop insurance. Billings says hail storms have already destroyed one of her family's greenhouses and several acres of their hemp crops this year, and without crop insurance all of that is simply time wasted and money lost. That heightened economic risk alone deters farmers from growing hemp and inhibits the industry's growth substantially.
The Hemp Industries Association's Lanier says that the industrial hemp sector will expand dramatically if McConnell's amendment becomes law, which could be a massive boon for the economy.
As the House and Senate hash out the final version of the farm bill and the DEA awaits the FDA's report, the entire cannabis industry, from farmers and retailers to patients and consumers, braces for impact, hopeful the politicians and bureaucrats will act in their favor.
"My prayer is that our leaders will understand that not only is this good for the economy and job creation," says Ramos, "it's also good for humanity."